How Research Leads to Cancer Treatment
Today’s cancer treatments have all gone through an extremely rigorous four-step testing process. This process is universal for all cancer treatments in the United States. In some cases, it leads to breakthrough drugs or therapies; other therapies are not approved for patient care.
After a new drug that shows promise for patients is developed in a lab, clinical trials begin to determine how effective and safe it is for humans. More patients are involved in the later phases of the trials.
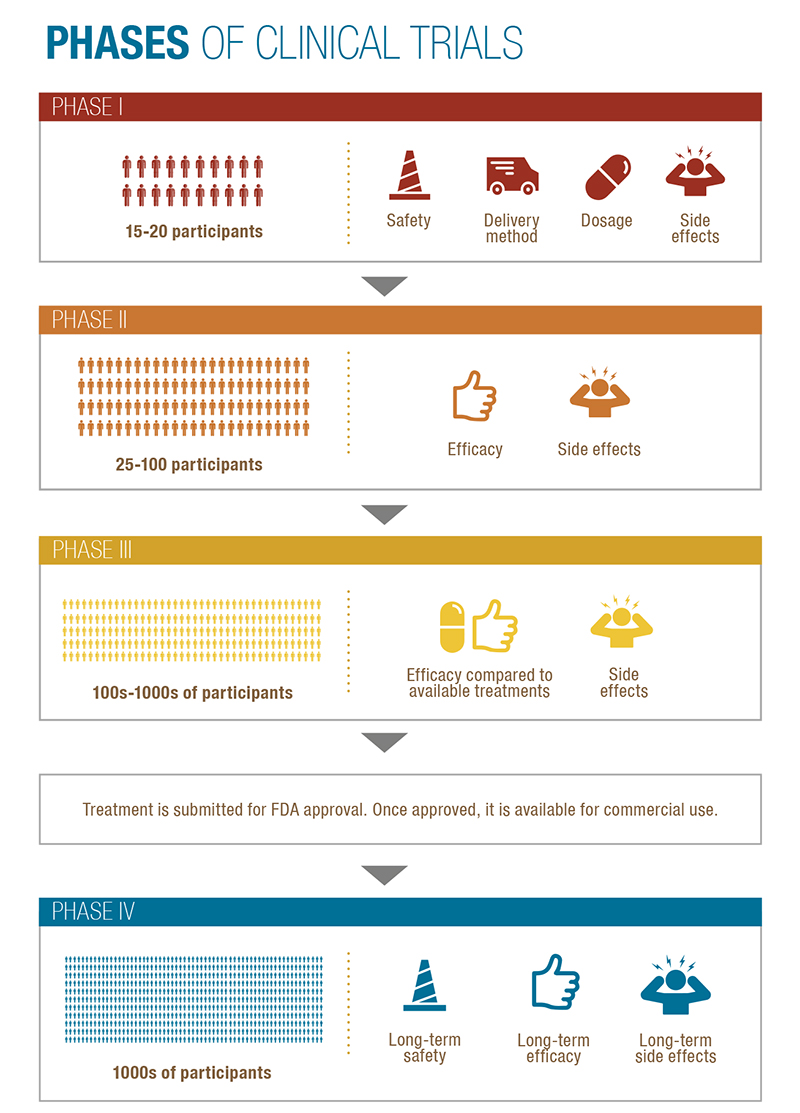
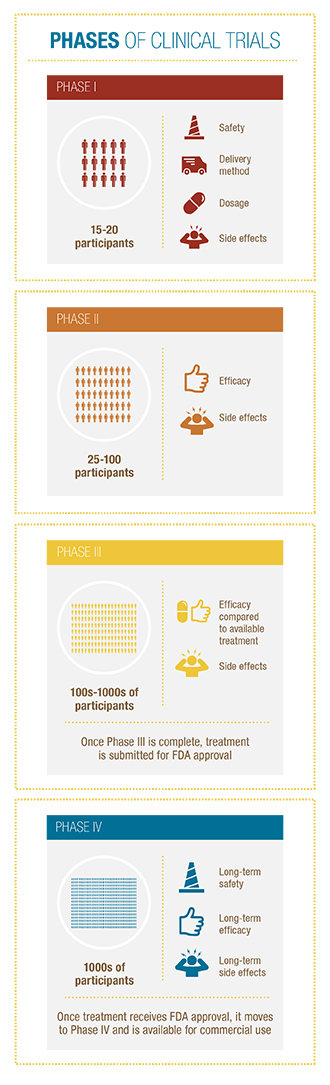
Clinical trials are conducted in four phases and, in some cases, lead to breakthrough drugs or therapies.
- Phase I Determines the dosage safety of a drug, the delivery method, and dosage frequency. (Approx. 15-50 participants)
- Phase II Examines the effectiveness of the treatment. (Approx. 25-100 participants)
- Phase III Compares a new drug or intervention with the current available treatment using randomly selected patients. (Approx. several hundred to several thousand people)
- After a treatment passes Phase III, it is submitted for approval by the Food and Drug Administration (FDA). Once the treatment is FDA approved, it is made available for commercial use.
- Phase IV examines the safety and effectiveness of a treatment over a longer period of time and among a wider patient population.
Research and Clinical Trials are an integral part of advancing cancer care. The physicians and staff at Texas Oncology are helping to advance cancer care in community practices with novel therapies.